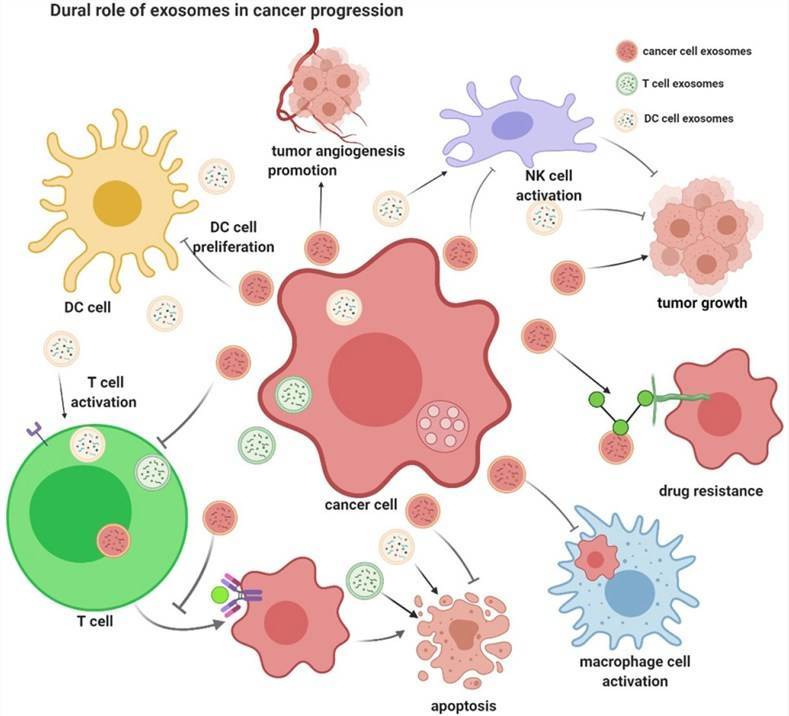
The Relationship Between Exosomes and Cancer
Exosomes are nanoscale vesicles derived from intracellular lysosomes that shuttle a variety of biomolecules, such as nucleic acids, proteins, lipids, amino acids and metabolites, and play a key role in regulating intercellular communication. It can be secreted by almost all types of cells, including immune cells, blood cells, neuronal cells, epithelial cells, and cancer cells. Exosomes contain various types of RNA molecules, including miRNA, mRNA, and even long non-coding RNA (lncRNA), which are significantly involved in the regulatory role of exosomes. Tumor cell-derived exosomes have important roles in reshaping the tumor microenvironment, promoting angiogenesis, metastasis and invasion, as well as regulating immune escape of tumor cells.

Roles of Exosome in cancer progression. (Von Schulze et al., 2020)
So, what is tumor microenvironment? The tumor microenvironment is the sum of cellular, molecular and physical environments surrounding the tumor, including tumor cells, immune cells, blood vessels, stroma, etc. The tumor microenvironment is closely related to tumor growth, metastasis, and drug resistance. In the tumor microenvironment, exosomes carrying tumor cell information can smoothly transmit them to other cells and directly participate in the regulation of tumor growth and metastasis. For example, exosomes play an important function in mediating intercellular information transfer between tumor cells and TAM in the tumor microenvironment, which may provide new targets for anti-tumor therapy. And tumor-associated macrophages (TAM) are among the most abundant immune cells in the tumor microenvironment.
How to Explore the Dynamics of Exosomal RNA in the Tumor Microenvironment
Exploring the dynamics of exosome RNA in the tumor microenvironment requires consideration of the following aspects.
Composition and types of exosome RNA
The composition and types of RNA of exosomes from different sources change. For exploring the dynamic changes of exosome RNA in the tumor microenvironment, the composition and trends of different sources and types of exosomal RNA need to be taken into account. For example, exosomal RNA released by tumor cells may contain some miRNAs related to tumorigenesis and progression, while exosomal RNA released by immune cells may contain some miRNAs related to immune regulation.
Secretion and uptake of exosome RNA
The secretion and uptake of exosomal RNA are important factors affecting the dynamics of exosomal RNA in the tumor microenvironment. When designing study protocols, researchers must account for how different pathways impact the uptake efficiency and biological function of exosomal RNA. Tumor cells and other cells can release exosomes through different pathways, such as through exocytosis, exothermic agglutination and release, and vesicular structures on the cell surface. In turn, cells that take up exosomal RNA can also do so through different pathways, such as endocytosis, fusion, receptor-mediated, etc.
Interaction of exosomal RNA with other molecules in the tumor microenvironment
The interaction of exosomal RNA with other molecules in the tumor microenvironment is also a key factor in exploring the dynamic changes of exosome RNA in the tumor microenvironment. Exosomal RNA can influence the function of other cells and molecules in the tumor microenvironment by regulating target gene expression, thus affecting tumor growth, metastasis, and immune escape. Therefore, researchers exploring dynamic changes in exosome RNA within the tumor microenvironment must account for its interactions with other molecules, their biological significance, and the underlying mechanisms of action.
Quantification and Analysis Methods of Exosomal RNA
The quantification and analysis methods of exosomal RNA are also key factors in exploring the dynamic changes of exosomal RNA in the tumor microenvironment. Currently, commonly used methods for quantifying and analyzing exosomal RNA include qPCR, sequencing, microarray, etc. All these methods have their advantages, disadvantages and applicability, and it is necessary to choose the appropriate method for quantification and analysis according to the specific research questions and experimental design. The more commonly used method is RNA sequencing, which can analyze the sequence and expression level of all RNA molecules simultaneously, including exosomal RNA. RNA sequencing can comprehensively analyze the composition and species of exosomal RNA. The advantage of RNA sequencing is that all RNA molecules can be detected, not limited to known sequences, and thus new exosomal RNA species and unknown biological functions.
Read our article Exosomal RNA Sequencing: Introduction, Categories, and Workflow to learn more about exosome RNA sequencing technologies.

CD Genomics Exosome RNA Sequencing Service
Tumor-Derived Exosomal RNA Is Involved in the Regulation of the Tumor Microenvironment
Many studies have demonstrated that tumor-derived exosomes play a crucial role in the tumor microenvironment for regulating macrophage polarity and mediating cancer-associated inflammation and tumor development.

Tumor microenvironment interactions. (Li et al., 2019)
Exosome miRNAs
In recent years, numerous studies have shown that exogenous miRNAs play a key role in tumor progression, stimulating angiogenesis and promoting metastasis. In addition, tumor-derived exosomal miRNAs polarize macrophages and target multiple signaling pathways, thereby positively or negatively influencing tumor progression. For example, CRC cells produce exosomes carrying miR-203 that incorporate into monocytes and polarize them to the M2 phenotype. Meanwhile, CRC cells also release exosomes containing miR-145 and miR-934, which macrophages take up to become M2. Additionally, colon cancer cells or gliomas secrete exosomes that deliver miR-1246 to induce a suppressive immune microenvironment. In addition, miRNAs in tumor cell-derived exosomes play an important role in regulating macrophage reprogramming and M1 polarization. For example, exosomes containing miR-130 or miR-33 increased the expression of M1 signature genes (IRF5, MCP1, CD80) and secretion of cytokines (IL-1β and TNF-α) in TAM, which inhibited tumor progression by converting M2 to M1 phenotype.
Exosome lncRNAs
New studies have shown that lncRNAs play key regulatory roles in tumorigenesis, macrophage activation, and polarization through different signaling pathways, and lncRNA-mediated crosstalk between TAM and cancer cells contributes to tumor progression.
For example, lncRNA RPPH1 has transferred from CRC cell-derived exosomes to macrophages, mediates M2 polarization of macrophages, increases expression of waveform proteins and Ki67, and decreases expression of E-calmodulin, thereby promoting metastasis and proliferation of cancer cells.
Exosome circRNA
Learn more: Radiant Skin
Exosome-Based Cancer Immunotherapy
Exosome-based therapies are emerging as a cutting-edge strategy to inhibit tumor progression or enhance anti-tumor immunity. Researchers have demonstrated that exosomes cross biological barriers like the blood-brain barrier, and they can modify these vesicles to boost efficiency. Thanks to their metastatic ability, lipid bilayer structure, and unique surface proteins, scientists use exosomes as nanoparticle carriers to deliver drugs, nucleic acids, and proteins directly to cancer cells.

Exosome-based cancer therapies. (Von Schulze et al., 2020)
Given the crucial role of macrophages in exosome-mediated cross-talk, the modulation of macrophages is an effective strategy for tumor therapy. Exosomes can be used as carriers of drugs and siRNAs to reverse M2 TAM-induced immunosuppression. In addition, reprogramming TAM to the M1 phenotype is another strategy. Mimetic nanovesicles derived from M1 macrophages actively convert M2 macrophages to the M1 phenotype in both in vitro and in vivo experiments. When administered intravenously in animal models, these vesicles inhibit tumor growth, and combining them with PD-L1 further enhances the antitumor effect.
Current therapeutic strategies focus on inhibiting the production of cancer cell-derived exosomes and blocking the uptake of specific exosomes by target cells. GW4869 is an inhibitor of exosome secretion and neutral sphingomyelinase that inhibits exosome production and achieves antitumor effects. Researchers loaded exosomes with chemotherapeutic agents like adriamycin and paclitaxel, and these nano-agents effectively inhibited cancer metastasis in mice.
References:
- Von Schulze, Alex, and Fengyan Deng. “A review on exosome-based cancer therapy.” Journal of Cancer Metastasis and Treatment 6 (2020): 42.
- Li, Irene, and Barzin Y. Nabet. “Exosomes in the tumor microenvironment as mediators of cancer therapy resistance.” Molecular cancer 18 (2019): 1-10.