Introduction
Medical writing plays a crucial role in modern healthcare by ensuring accurate, clear, and well-structured communication of medical and scientific information. It bridges the gap between healthcare professionals, regulatory bodies, researchers, and patients, facilitating the dissemination of knowledge essential for medical advancements and patient care. Whether in research documentation, regulatory submissions, medical journalism, or patient education, medical writing is indispensable to the healthcare ecosystem.
Definition
Medical writing is the process of producing scientific publications that clearly and accurately convey medical, clinical, or pharmaceutical information. It entails creating a wide variety of content, including clinical study reports, regulatory submission paperwork, research papers, medicine labels, patient information leaflets, and medical journal articles. Medical writers must ensure that their materials are exact, well-organised, and adhere to regulatory guidelines.
The Scope of Medical Writing
Medical writing encompasses a wide range of disciplines, including clinical research, regulatory affairs, medical education, and healthcare marketing. It involves writing scientific documents such as clinical trial reports, research articles, patient information leaflets, and promotional materials for pharmaceutical and medical device companies. The increasing complexity of medical science and the growing demand for regulatory compliance have expanded the role of medical writers in healthcare.
1. Regulatory and Clinical Writing
Regulatory writing is one of the most vital aspects of medical writing. It involves the preparation of documents required for regulatory approval of drugs, medical devices, and biologics. These documents include:
- Clinical Study Reports (CSRs)
- Investigational New Drug (IND) applications
- New Drug Applications (NDAs)
- Common Technical Documents (CTDs)
Regulatory writers ensure that documents comply with the guidelines set by regulatory authorities such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Council for Harmonisation of Technical Requirements for Human Use Pharmaceuticals (ICH).
2. Scientific and Research Writing
Scientific medical writing involves composing research papers, systematic reviews, case reports, and abstracts for peer-reviewed journals. This type of writing is crucial in disseminating new scientific discoveries, clinical findings, and medical innovations. Researchers rely on skilled medical writers to present complex data in a structured and comprehensible manner, ensuring clarity and accuracy in scientific literature.
3. Medical Journalism
Medical journalism focuses on translating complex medical and scientific information into engaging and accessible content for the general public. This includes writing articles for health magazines, newspapers, and online platforms that educate people about diseases, treatments, healthcare trends, and wellness. Medical journalists help in debunking myths and spreading awareness about various health issues.
4. Medical Education and Training Materials
Medical writing also plays a significant role in creating educational content for healthcare professionals and students. This includes writing medical textbooks, e-learning modules, clinical guidelines, and Continuing Medical Education (CME) materials. Well-structured and evidence-based educational resources contribute to the ongoing professional development of healthcare providers.
5. Healthcare Marketing and Communications
Pharmaceutical and healthcare companies rely on medical writers to create promotional materials, brochures, and advertisements that comply with medical ethics and regulatory standards. This form of writing helps in branding, patient engagement, and communicating the benefits of medical products and services in a credible manner.
The Importance of Medical Writing in Healthcare
Enhancing Patient Education and Awareness:
Medical writing plays a crucial role in patient education by developing easy-to-understand health content such as patient information leaflets, disease awareness brochures, and online health blogs. These materials help patients make informed decisions about their health and treatment options.
Ensuring Regulatory Compliance:
Regulatory medical writing ensures that pharmaceutical and medical device companies meet the necessary legal and ethical requirements. Accurate documentation is essential for obtaining approvals from regulatory bodies, thus facilitating the availability of safe and effective treatments to the public.
Bridging the Gap Between Research and Practice:
Medical writers help translate complex scientific findings into practical knowledge that can be applied in clinical settings. By summarizing research outcomes in an accessible format, they assist healthcare providers in staying updated with the latest medical advancements.
Supporting Clinical Trials and Drug Development:
Accurate documentation in clinical research is crucial for the successful development of new drugs and therapies. Medical writers prepare essential clinical trial documents that help in monitoring patient safety, analyzing treatment effectiveness, and obtaining regulatory approvals.
Maintaining Ethical and Accurate Communication:
Medical writing ensures that all healthcare-related content adheres to ethical standards and maintains scientific integrity. Well-researched and properly referenced documents reduce the risk of misinformation and enhance trust in medical literature.
The Skills Required for Medical Writing
A professional medical writer must possess a combination of scientific knowledge, writing proficiency, and an understanding of regulatory requirements. Essential skills include:
- Strong grasp of medical and scientific concepts
- Ability to simplify complex information for different audiences
- Attention to detail and accuracy
- Knowledge of medical terminologies and guidelines
- Proficiency in literature research and data analysis
- Compliance with ethical and regulatory standards
Many medical writers have backgrounds in life sciences, medicine, or pharmacy. Specialized training and certifications, such as those offered by the American Medical Writers Association (AMWA) or the European Medical Writers Association (EMWA), enhance credibility in the field.
The Future of Medical Writing
The demand for medical writers is growing as the healthcare industry continues to expand. Emerging trends in medical writing include:
- Artificial Intelligence (AI) and Automation: AI-driven tools assist in data analysis, document drafting, and regulatory writing, increasing efficiency and accuracy.
- Personalized Medicine: The rise of genomics and personalized healthcare demands more tailored medical documentation.
- Digital Health Communication: With the proliferation of telemedicine and health apps, there is a need for high-quality digital health content.
- Data Transparency and Open Access Publishing: More emphasis is being placed on making scientific research accessible to the public through open-access journals and transparent reporting.
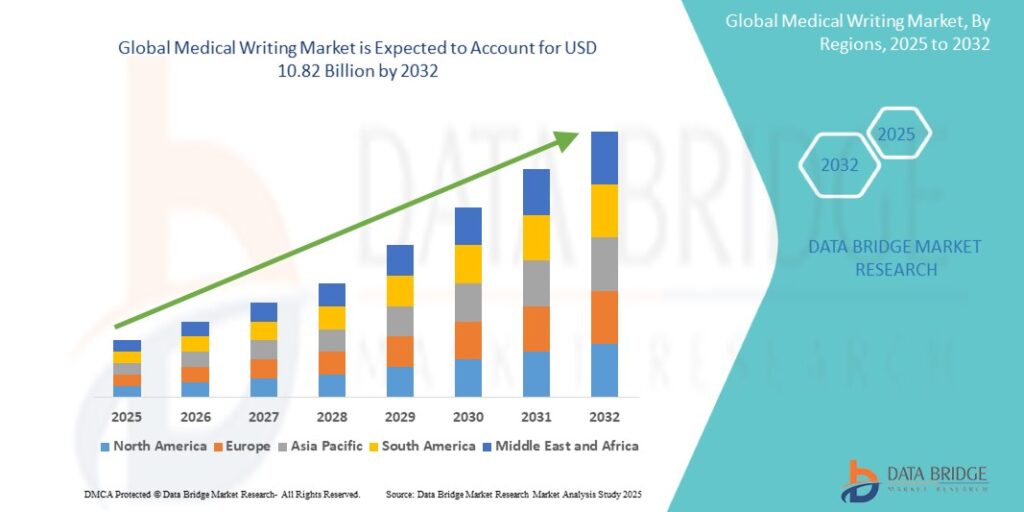
Growth Rate of Medical Writing Market
According to Data Bridge Market Research, the medical writing market was valued at USD 4.70 billion in 2024 and is anticipated to reach USD 10.82 billion by 2032, with a CAGR of 10.98% from 2025 to 2032.
Read More: https://www.databridgemarketresearch.com/reports/global-medical-writing-market
Conclusion
Medical writing is an essential pillar of modern healthcare, facilitating clear communication between researchers, healthcare professionals, regulatory authorities, and patients. It ensures accurate dissemination of medical knowledge, supports clinical research, and enhances patient education. As the healthcare landscape continues to evolve, the role of skilled medical writers will remain critical in bridging the gap between science and society, ultimately contributing to better healthcare outcomes.